New Thermochemical Energy Storage Material for Energy Storage in Buildings
Impregnating a crystalline nanocellulose framework with calcium chloride provides a new stable thermal material for recycling solar, waste or other heat sources for improved building energy efficiency.
Background:
In 2018, nearly 17 quadrillion BTUs were consumed by US buildings for heating. The majority of this energy has been non-renewable sourced. Renewable sources or waste heat could meet a significant part of this need if effective thermal storage were available to facilitate the time-shift from when the energy is available to when it is needed.
Hygroscopic salts have been proposed for thermochemical energy storage. The reaction works by dehydrating the salt during the charging phase. This is an endothermic reaction. When energy is needed, water vapor is passed by the salt to hydrate it. This is an exothermic reaction which releases the stored energy. However, these salts are not stable over repeated charging and discharging (dehydrating-hydrating). Various porous media stabilizing agents have been used to improve stability, but these approaches result in decreased energy density of the salts and plugging of the porous material. The lack of an optimal storage structure has limited the deployment of commercially available energy storage systems. Montana State University and North Dakota State University researchers have developed a new composite material consisting of a combined crystalline nanocellulose (CNC) and CaCl2 salt framework that overcomes the existing challenges of currently proposed hygroscopic salts for energy storage.
Solution:
The new energy storage material overcomes the stability challenges of hygroscopic salts by impregnating the salts in cellulose nanocrystals (CNC). CNCs are extremely versatile crystalline structures obtained in a variety of ways from natural cellulose. CNCs are used as stabilizing structure in a variety of applications including pharmaceutical, separation membranes and electronic components. Impregnating CNCs with salts provides a bio-based sustainable framework of significantly smaller salt particles to improve surface area, reaction rates and reduce salt degradation. The CNCs overcome agglomeration which occurs with pure salts and the CNCs minimize plugging found with other hygroscopic salt stabilization methods such as impregnating the salts in foam. The CNCs are hydroscopic and absorb water which can lower the dehydration temperature below other salt stabilizing structures.
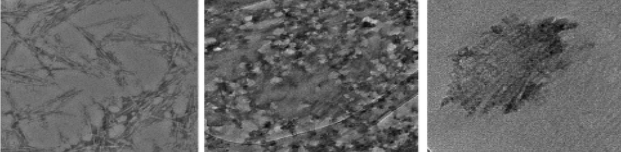
Figure 1: TEM micrograph (a) Cellulose nanocrystals (b) CaCl2 salt particles (c) Combined CNC and CaCl2 particles showing complex tangled network (dark spots show some agglomeration).
Benefits:
- Stability over repeated heat storing and discharging
- Minimal plugging of the thermochemical energy storage material
- Operates at lower temperatures
Opportunity:
- Pending patent is available for license
- Research is ongoing and researchers are available for collaboration
Supporting publications:
-
Adam Gladen and D.S. Bajwa. A Novel Composite Material of Hygroscopic Salt Stabilized by Nanocellulose for Thermochemical Energy Storage. Energy Sustainability. ES2021-63814, V001T06A006, https://doi.org/10.1115/ES2021-63814
Contact:
Nick Zelver
406‐994‐7706
nzelver@montana.edu
